Research
Current Main Projects
Desynchronizing weak
E-fields during DBS
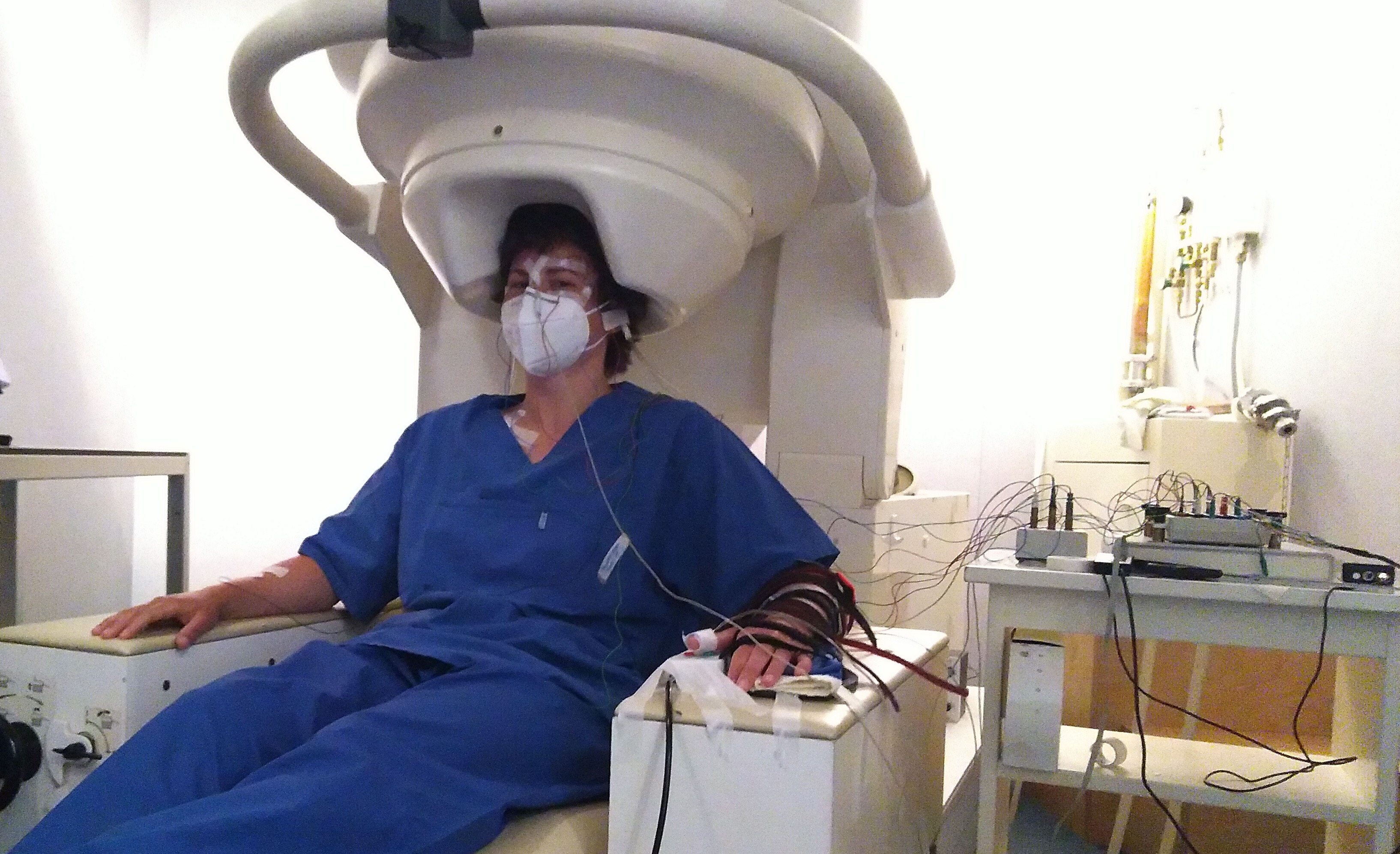
The project DECODE, funded by the European Research Council (ERC), investigates the influence of weak cortical E-fields on the therapeutic outcome of deep brain stimulation. We use a combination of E-field modeling (PhD project of Thomas Keizers), computational neuroscience, EEG measurements in healthy participants, and clinical recordings. For a general description, see this U-Today article.
We are looking for an additional PhD student or Postdoc as well as a Medical Doctor in this project, please see Join Us.
Main collaborations: Andrew Sharott (University of Oxford, UK), Monika Pötter-Nerger (Department of Neurology, UKE), Hil Meijer (Department of Mathematics, UT), Maria Carla Piastra (Clinical Neurophysiology, UT).
Advisory board: Tim Denison (University of Oxford, UK), Andrew Sharott (University of Oxford, UK), Massimo Sartori (Neuromechanical Engineering, UT).
Ethics Advisor: Dorothee Horstkoetter (University of Maastricht, NL).
Model-based optimization
of dual-site tACS
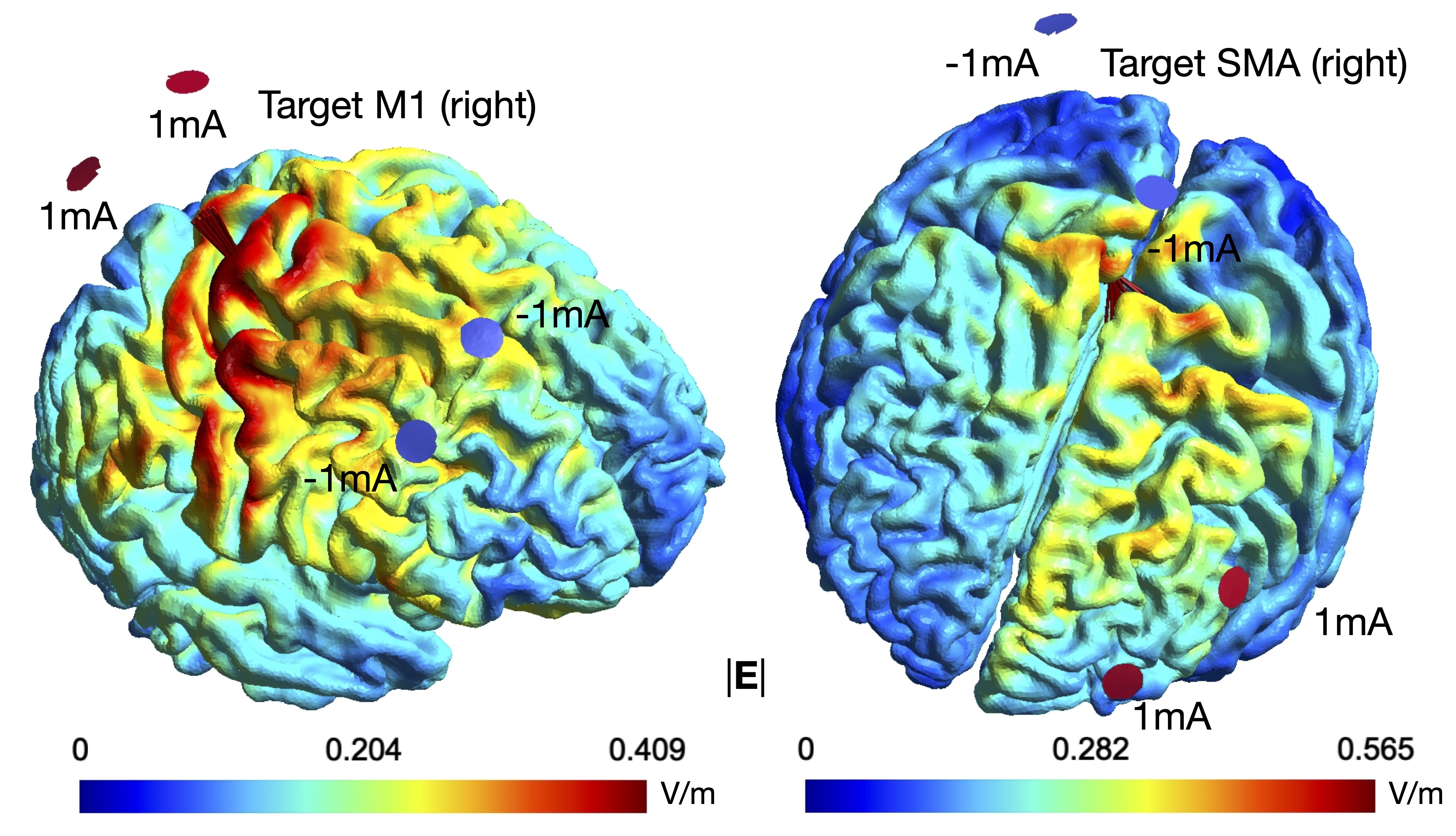
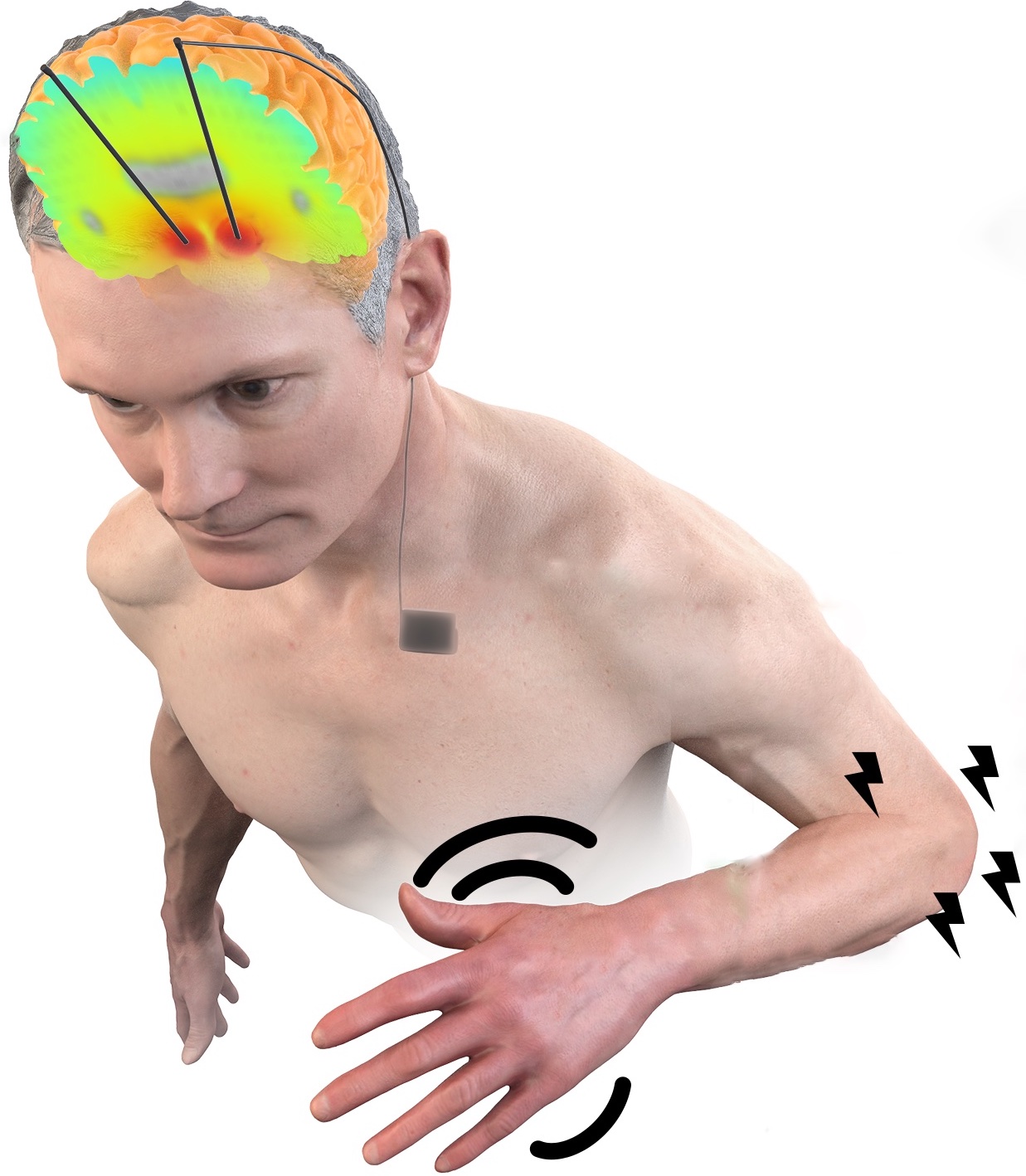
PhD student Silvana Huertas Penen investigates connectivity modulation in the motor system by dual-site transcranial alternating current stimulation (tACS), and how computational models may help to personalize stimulation.
Simulations and experiments with healthy participants are conducted at the University of Twente and the Donders Institute for Brain, Cognition and Behaviour (Nijmegen) in collaboration with Ivan Toni.
Multi-modal network stimulation
in Parkinson’s disease
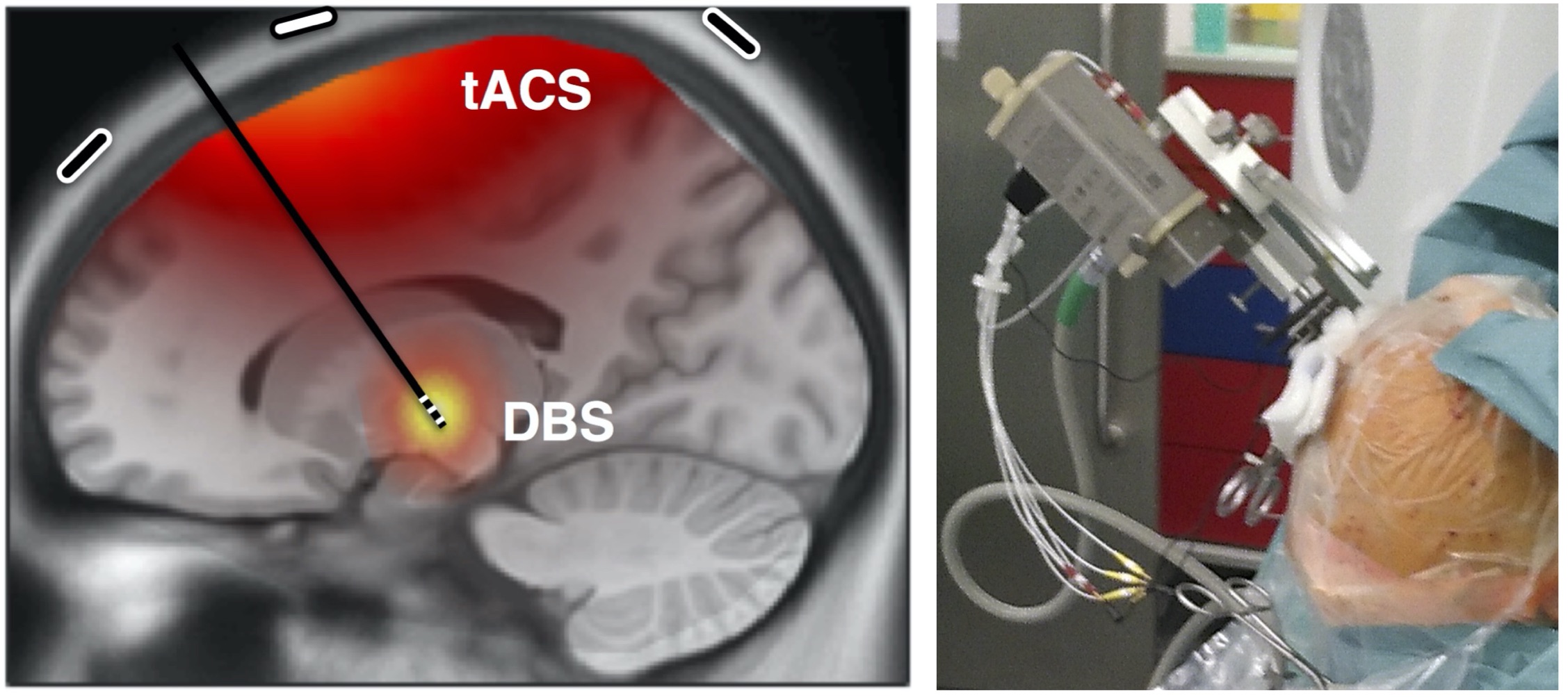
In this project at UKE Hamburg funded by the German Research Foundation (DFG), in collaboration with the University of Oxford (UK), postdoc Mareike Gann studies the combination of transcranial alternating current stimulation (tACS) and deep brain stimulaion (DBS) coupled in a brain-computer interface to target cortico-subcortical connectivity. A further part of the project is on the basics of tACS and dual-site tACS to modulate functional connectivity.
Main collaborations: Christian Moll (neurophysiology, UKE), Wolfgang Hamel (neurosurgery, UKE), Monika Pötter-Nerger (neurology, UKE), Alessandro Gulberti (neurophysiology / neurology, UKE), Andrew Sharott (University of Oxford, UK), Tim Denison (University of Oxford, UK), Charlotte Stagg (University of Oxford, UK).
Previous Projects
Phase-specific
modulation of neural
activity by tACS
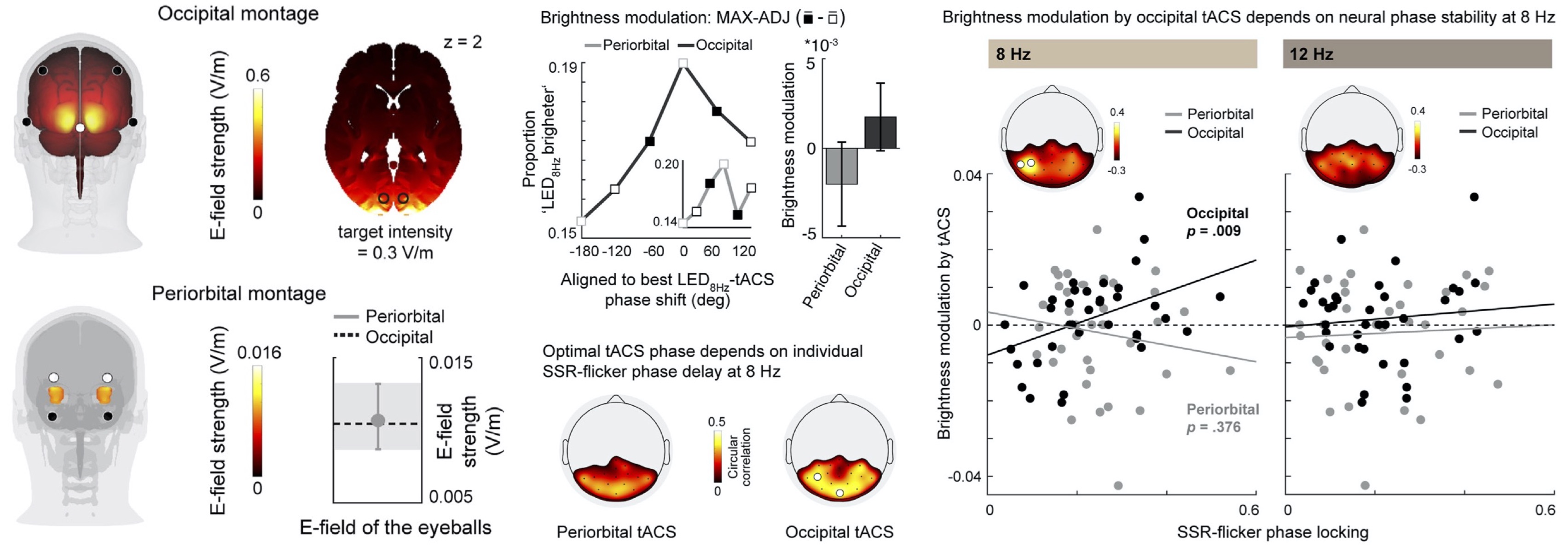
PhD student Marina Fiene (Andreas Engel Lab, UKE Hamburg) investigated how transcranial alternating current stimulation (tACS) can phase-specifically influence flicker-evoked EEG activity as well as related behavior. Both EEG activity and subjective brightness perception showed dependencies of this modulation on baseline flicker entrainment metrics, thus emphasizing the need for personalized stimulation settings.
Connectivity
modulation by
dual-site tACS
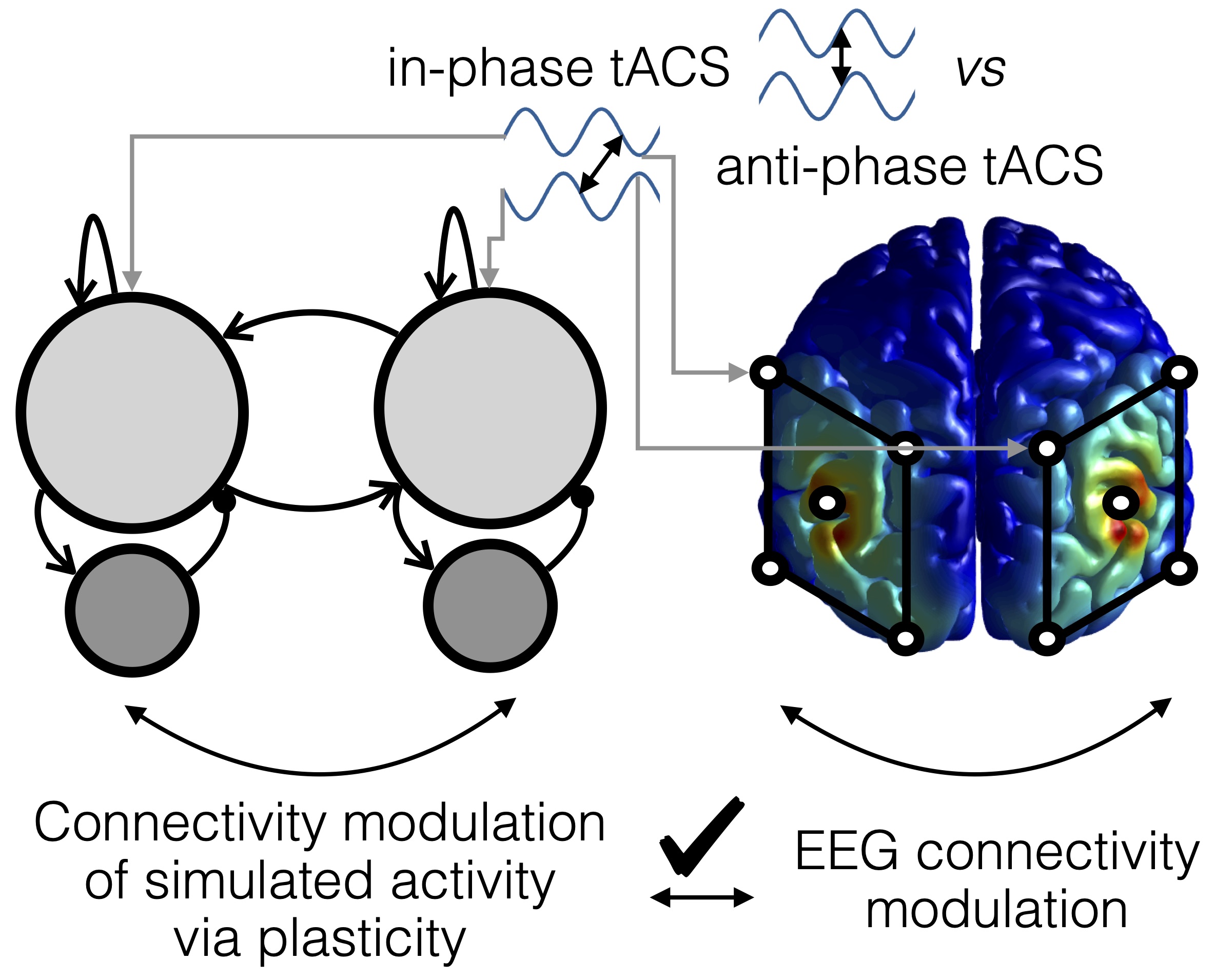
We (Andreas Engel Lab, UKE Hamburg) were able to show that transcranial alternating current stimulation (tACS), applied at two brain sites (dual-site), can modulate functional connectivity in the alpha range, with a moderate specificity in space and frequency. The tACS montage was here tuned in a way to yield no differences in applied electric field strength and thus alpha power across our conditions, while the direction of electric fields differed across conditions to induce connectivity changes.
Furthermore, we used a neural nework model demonstrating that synaptic plasticity can account for the described connectivity changes, possibly in interaction with entrainment echoes.
Functional connectivity of
basal ganglia and thalamus
during movement
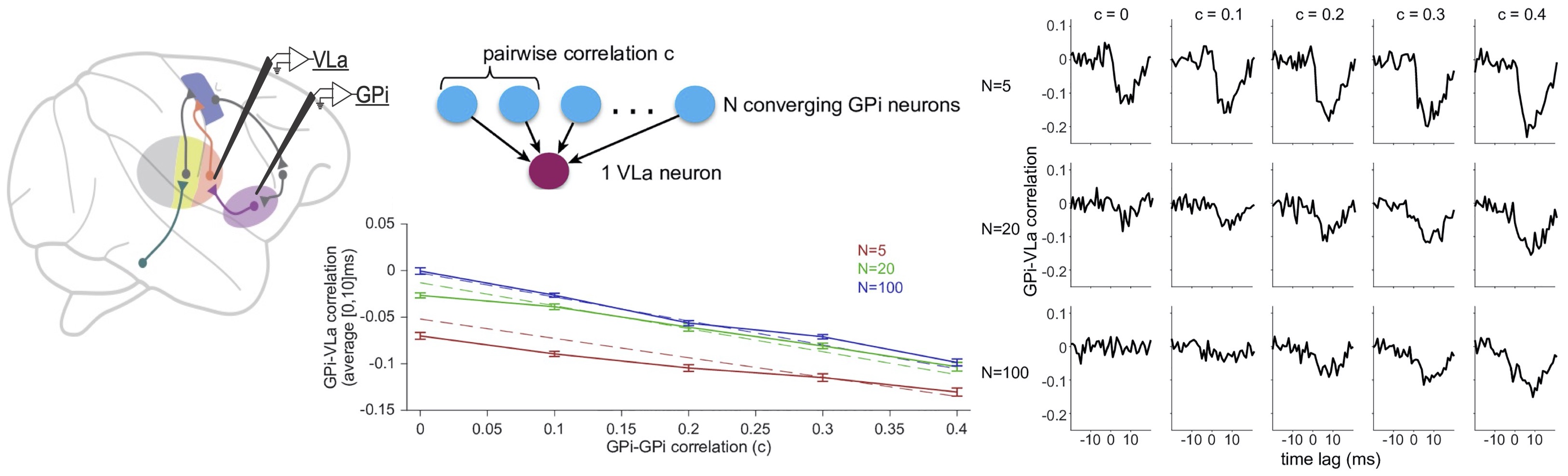
With paired unit recordings from output nuclei of the basal ganglia and input nuclei of thalamus, we (Robert Turner Lab & Jonathan Rubin Lab, University of Pittsburgh, US) found low functional connectivity of basal ganglia and thalamus during rest and during learned movement. We suggest that synchrony in the basal ganglia may be critical to render the pathway effective in both physiological and pathophysiological situations.
All experiments were done by the Robert Turner Lab, University of Pittsburgh, PA, USA.
Synchronization of
neural activity
in the basal ganglia
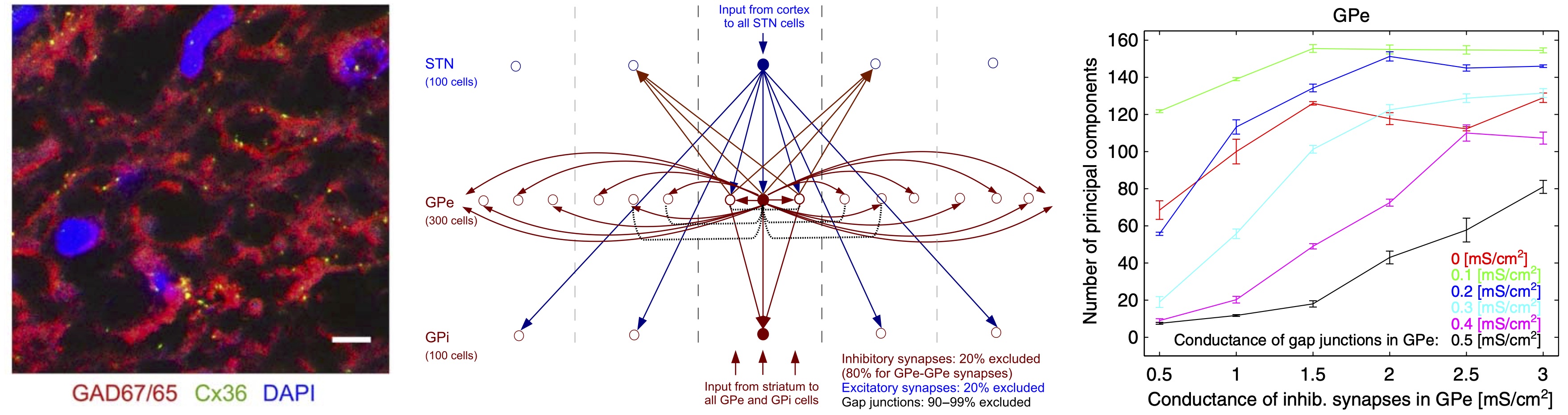
Why does neural activity in the basal ganglia of individuals with Parkinson’s disease synchronize? The globus pallidus may play a critical role here. We (Richard van Wezel Lab, Donders / University of Twente; Stephan van Gils Lab, University of Twente) found Cx36 in the external and internal pallidum, and described how gap junctions between neurons in the pallidum affect synchrony in a network model of the basal ganglia.
Quantification of
cardiac electrophysiology
models
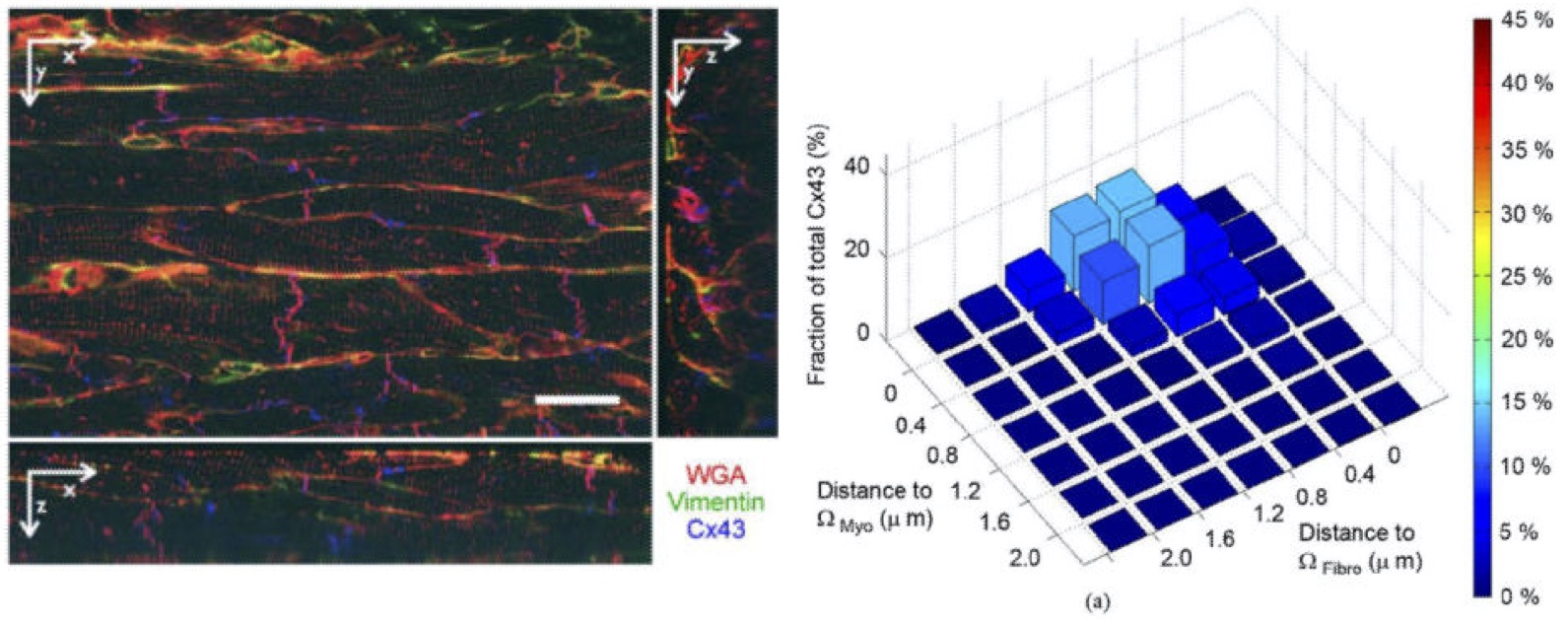
Cardiac dynamics are determined by properties on a micro- and macro-scale, and can successfully be simulated with the combination of cellular and macroscopic models. However, many parameters on both scales, but in particular on the microscale, are not known. Thus, we (Frank Sachse Lab, University of Utah, US; Gunnar Seemann Lab, Karlsruhe Institute of Technology, Germany) extracted quantitative parameters from cardiac tissue using three-dimensional confocal imaging and image reconstruction. Critically, we found that the distribution of Cx43, potentially forming gap junctions, changed with myocardial infarction.
Copyright © 2024 Bettina C. Schwab.